Unlocking Breakthroughs:
8 Ways DVS is Essential to
Pharmaceutical Research
Moisture sorption properties stand at the forefront of pharmaceutical material considerations, exerting profound effects on storage, stability, processing, and performance. Guided by the stringent standards of regulators around the world, moisture, far from being a mere impurity, commands strict monitoring and control, especially within drug substances.
The implications of moisture content extend across critical facets of pharmaceutical products, influencing crystallinity, storage modulus, permeability, density, and the melting point, with a pronounced impact on amorphous materials. Recognizing the need for a nuanced approach to moisture analysis, the Dynamic Vapor Sorption (DVS) technique was developed by Surface Measurement Systems in the early 1990s.
This technique, now a cornerstone in pharmaceutical research, offers a rapid, highly-sensitive, and automated method for studying moisture sorption properties across a spectrum of materials. Below we unravel the multifaceted benefits of employing DVS in the analysis of pharmaceutical materials.


1. Compliance with pharmaceutical regulations
The Dynamic Vapour Sorption (DVS) technique is instrumental in meeting stringent US pharmaceutical standards by providing a rapid, highly sensitive, and continuous approach to studying moisture sorption properties. Developed by Surface Measurement Systems, the DVS instrument ensures compliance with US Pharmacopeia guidelines by actively measuring and controlling relative humidity and a wide range of organic vapour concentrations. This precision in environmental control enhances understanding across various applications, including the determination of moisture content, sorption isotherms, and the characterization of hydrates and solvates. Additionally, DVS proves valuable in assessing hygroscopicity, a crucial factor in preformulation activities and the selection of drug crystal forms.

2. Provides unparalleled insight into moisture-induced phase changes
Understanding moisture-induced phase changes is vital in the development of pharmaceutical materials, with a focus on crystallinity, amorphous substances, and hydrate and solvate formation. DVS enables precise examination of amorphous solids, crucial for understanding the critical humidity at which glass transitions occur, impacting storage and processing conditions. Linear relative humidity ramping experiments and 2-dimensional phase diagrams contribute to determining optimal stability conditions for amorphous pharmaceutical ingredients.
Furthermore, DVS plays a vital role in characterizing hydrate and solvate formation, offering valuable data for pharmaceutical development. It detects different hydrate forms and studies stoichiometric solvates, providing a versatile methodology for various solvent concentrations. The integration of DVS with in-situ vibrational spectroscopy enhances the understanding of moisture-induced phase changes by monitoring molecular vibrational characteristics. In summary, DVS is an indispensable tool for unravelling the complexities of moisture-induced phase changes in pharmaceuticals, encompassing crystallinity, amorphous materials, and hydrate and solvate formation.
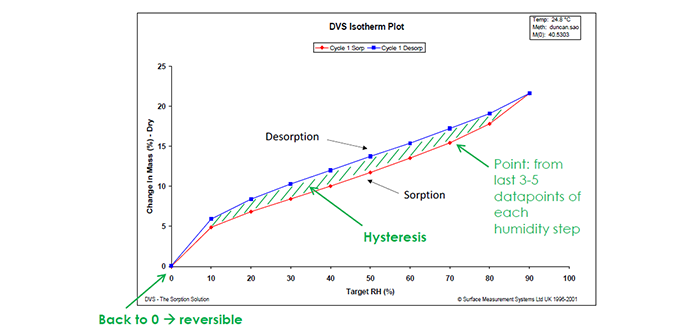
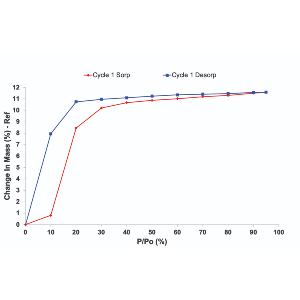
3. Understand the moisture sorption hysteresis of your materials
When attempting to analyse moisture sorption hysteresis, the DVS technique has a unique ability to swiftly measure sorption kinetics and determine moisture diffusion coefficients, facilitating a comprehensive understanding of material transformations. This rapid and continuous measurement capability is in stark contrast to the prolonged timelines associated with traditional desiccator jar methods. DVS’s role extends beyond the laboratory, offering insights into formulation optimization, manufacturing, and quality control processes. By studying moisture sorption hysteresis, researchers can make informed decisions about storage requirements, ensuring the stability and performance of pharmaceutical materials. The instrument’s simultaneous determination of sorption and desorption isotherms on the same sample is particularly valuable, shedding light on dynamic material behaviour under varying humidity conditions. This nuanced understanding aids in tailoring formulations for optimal performance, ultimately contributing to the efficiency and quality of pharmaceutical production.

4. More than just powders – gain vital insight on pharma packaging materials
Dynamic Vapour Sorption (DVS) emerges as an indispensable tool in pharmaceutical research, particularly in the realm of studying packaging materials. DVS facilitates the measurement of diffusion coefficients for films, powders, and fibers, providing valuable insights into the permeability and transport properties of pharmaceutical packaging materials. By conducting real-time mass change experiments, DVS allows for the determination of diffusion coefficients over a range of temperatures, offering a nuanced understanding of how these materials interact with water vapor. This capability is particularly beneficial for packaging applications, where the diffusion into films can be critical for ensuring the stability and integrity of pharmaceutical products during storage and transportation.
Furthermore, DVS proves instrumental in evaluating water vapor transmission rates (WVTR) for pharmaceutical packaging materials. The innovative Payne style diffusion cell, designed for this purpose, enables the measurement of the rate of diffusion of water vapor through thin films. This information is crucial for assessing the effectiveness of packaging barriers in preventing moisture ingress, which is vital for maintaining the quality and shelf-life of pharmaceutical products. The combination of diffusion coefficient determination and WVTR measurement using DVS provides comprehensive data for optimizing pharmaceutical packaging, contributing to the development of packaging solutions that meet stringent quality standards and regulatory requirements.

5. Gain detail insight into sorption mechanisms of pharma materials
Having a detailed understanding of the sorption mechanisms of any given material is essential in determining the effective use of pharmaceutical materials, including excipients, drug formulations, and packaging films. One significant benefit of DVS is its ability to generate moisture sorption isotherms, providing crucial insights into the material’s response to varying relative humidity (RH) levels. Unlike traditional methods such as the desiccator jar, DVS enables continuous measurement of sorption kinetics, allowing for the determination of moisture diffusion coefficients and the measurement of sorption and desorption isotherms on the same sample. This real-time capability significantly accelerates the process, reducing the time required for determining isotherms from weeks or months to hours or days. Additionally, DVS facilitates the measurement of moisture-induced phase changes, aiding in the understanding of critical humidity thresholds for glass transitions and potential issues such as powder caking.
As previously mentioned, the DVS technique proves instrumental in characterizing hydrates and solvates of pharmaceutical materials. It enables the detection and characterization of hydrate formation based on environmental relative humidity, allowing for a deeper understanding of the influence of hydration states on physico-chemical properties. DVS can also extend its applicability to studying stoichiometric solvates, providing valuable information on solvate formation and stability. The technique’s combination with in-situ vibrational spectroscopy enhances its capabilities, allowing for the monitoring of molecular vibrational characteristics during hydration state changes. Overall, DVS emerges as a powerful tool for predicting dispersion in liquid solvents, offering insights into sorption kinetics, isotherm characteristics, and the formation of hydrates and solvates critical for pharmaceutical development and quality control.
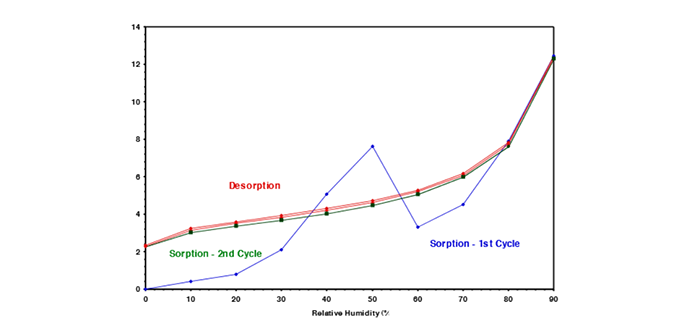
6. Understand the importance of sorption and desorption curves with continuous measurement of sorption kinetics
Crucial insights into moisture sorption properties through the analysis of sorption and desorption isotherms are vital to ensuring stability in materials for new coatings and treatments. By continuously measuring sorption kinetics in real-time, DVS enables the determination of moisture diffusion coefficients and the exploration of moisture sorption hysteresis on the same sample. This dynamic approach accelerates data collection, offering a nuanced understanding of how pharmaceutical materials interact with water vapor at various relative humidity levels, essential for assessing storage conditions and preventing potential degradation risks.
Sorption kinetics data obtained from DVS play a pivotal role in developing coatings and treatments aimed at maintaining product stability. Understanding the rate of moisture absorption and release empowers researchers to design precise coatings, preventing moisture-induced phase changes that could compromise the stability of amorphous pharmaceutical ingredients. DVS’s real-time, detailed sorption kinetics data facilitate the customization of coatings and treatments, ensuring materials withstand environmental humidity, contributing to the formulation of pharmaceutical products that meet stringent stability and quality requirements throughout their lifecycle.

7. Impact of moisture in Hydrolysis and Drug Degradation
Crucial insights into moisture sorption properties through the analysis of sorption and desorption isotherms are vital to ensuring stability in materials for new coatings and treatments. By continuously measuring sorption kinetics in real-time, DVS enables the determination of moisture diffusion coefficients and the exploration of moisture sorption hysteresis on the same sample. This dynamic approach accelerates data collection, offering a nuanced understanding of how pharmaceutical materials interact with water vapor at various relative humidity levels, essential for assessing storage conditions and preventing potential degradation risks.
Sorption kinetics data obtained from DVS play a pivotal role in developing coatings and treatments aimed at maintaining product stability. Understanding the rate of moisture absorption and release empowers researchers to design precise coatings, preventing moisture-induced phase changes that could compromise the stability of amorphous pharmaceutical ingredients. DVS’s real-time, detailed sorption kinetics data facilitate the customization of coatings and treatments, ensuring materials withstand environmental humidity, contributing to the formulation of pharmaceutical products that meet stringent stability and quality requirements throughout their lifecycle.
8. Unlock advanced applications with organic solvents sorption analysis
When applied with organic solvents, the Dynamic Vapour Sorption (DVS) technique unlocks advanced applications that significantly contribute to characterizing and determining physicochemical properties, particularly surface area, in pharmaceutical materials. DVS instruments, with their unique capability to actively measure and control the concentration of a broad range of organic vapours, extends its utility beyond water vapour analysis. This innovative feature allows researchers to explore the interaction between pharmaceutical materials and organic solvents in real time. Notably, the DVS technique applied to organic solvents proves instrumental in determining surface area using the BET (Brunauer-Emmett-Teller) method. Unlike traditional volumetric techniques, DVS experiments are performed at atmospheric pressure and room temperature, mitigating the risk of altering the fragile structure of materials.
The application of DVS with organic solvents is exemplified in the determination of the BET surface area for pharmaceutical materials, such as Metformin Hydrochloride, showcasing the technique’s advantages over conventional methods. The dynamic flow nature of DVS enables rapid equilibration, providing efficient and accurate measurements of surface area with relatively small sample sizes. This capability is particularly advantageous for new drug entities or materials with very low surface areas. Therefore, the DVS technique, when coupled with organic solvents, emerges as a powerful tool for pharmaceutical researchers, offering a dynamic and precise approach to surface area determination and advancing the characterization of materials critical for drug development and quality control.

Conclusion:
In conclusion, the Dynamic Vapour Sorption (DVS) technique presented here demonstrates its pivotal role in unraveling the complexities of moisture sorption, hydrate/solvate formation, and physicochemical properties in pharmaceutical materials. Its wide range of applications, from understanding moisture-induced phase changes to characterizing surface area with organic solvents, showcases the versatility and precision of DVS technology.
For those seeking to delve deeper into these advanced applications and explore the full potential of our DVS, find out more about our products on our company page, or read a full Pharma Overview Scientific Note for DVS using the link below.