The effect of outgassing temperature on H₂O & CO₂ adsorption performance of MOFs & Zeolites
Watch now
Abstract:
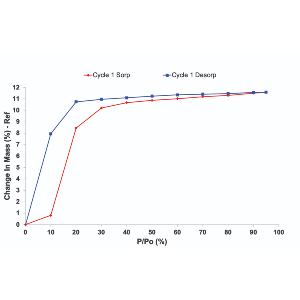
Crystalline solids with controllable structures possess tailored porosity and large surface areas. This is particularly attractive for gas storage and separation applications. Physisorption of gases is a technique applied for the characterization of porous solids, such as zeolites and metal-organic frameworks (MOFs). Gravimetric vapor sorption and gas adsorption techniques measure promising functionalities such as removal of carbon dioxide (CO2) from the atmosphere or stability of sorbents in humid environments. Currently, both techniques are routinely applied for the characterization of porous solids to explore adsorption capacities and porosity. However, only a few studies focus on the pre-experimental conditions for the determination of gas/vapor sorption isotherms. Details of outgassing conditions, despite their importance, are often lacking in research publications. Outgassing at low temperatures of thermally stable material provides an incomplete cleaning of the porous surface. As a result, the ability of sorbents to store CO2 or water molecules is underestimated based on adsorption data. Contrary, outgassing of temperature-sensitive sorbent at elevated temperatures can cause irreversible structural changes which will have profound effects on the adsorption capacities. The impact of water adsorption on the structure was isolated by introducing partial pressures of water under a vacuum. Such measurements provided a true water adsorption isotherm without unnecessary interference from a carrier gas. CO2 adsorption data were measured from low pressures up to 1bar. CO2 adsorption in the presence of water on the sorbent was collected in a system under a vacuum. The results show that the performance of the sorbent can be significantly modified depending on outgassing conditions.
Dr. Vladimir Martis
Product Manager
Surface Measurement Systems